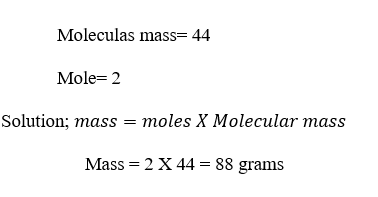Molecular mass: it is defined as the total mass of atoms that dwell in molecules.
For
example; if you are asked to calculate the molecular mass of CO2 then
we will it as following?
Solution;
C=12x1=12
O=16 x2=32
44 (a.mu)
Moles:
The moles are defined as the atomic masses, formula mass, and molecular mass
expressed in grams.
Example
1; calculate the number of moles in 22g of CO2?
Example
2; Calculate the mass of 2 moles of CO2?
DATA; Mass=??
Formula
mass: The total sum of the atomic
masses of the atoms present in
the empirical formula of
the compound is called formula mass.
Example;
calculate the formula mass of NaCl?
Solution; Na=23 X1=23
Cl= 35X1=35
58 (a.m.u)
Avogadro’s number:
The Avogadro’s is defined as the one mole of any substance contains 6.02X10-23
it represented by NA. Avogadro’s number is used to calculate the
number of atoms or calculate the no of molecules.
For
example; calculate the number of atoms of Al in 22 g?
Data;
no; of atoms=??
Given mass= 22 g
Molecular mass= 27
NA= 6.02X10-23
Molecular formula
The
formula which shows the actual number of molecules is known as the molecular
formula. For instance, C6H6 is the molecular
formula of benzene.
Empirical formula:
The
formula which does not shows the actual number of molecules only shows the
simplest ratio is known as the empirical formula. For instance, CH is the
empirical formula of benzene whose chemical formula is (C6H6).
Conservation of mass:
The
mass of the reactant before the reaction and the mass of the product after the reaction
remains the same. The initial weight of
the reactant and final weight of the product remain fixed reaction may be
changed. For example, H2O—> H+OH the mass of water before the
reaction is 18 after the decomposition of water the mass remains 18. from the
above example it is concluded that the reaction may be changed but the mass
remains constant.
Atomic mass:
Atomic
mass is defined as the sum of the total number of protons and neutrons. It is
denoted by’ A’. The mathematical formula of atomic mass is A=N+Z.
Atomic number
The
number of protons present in an atom. For instance, 6 is the atomic number of the
carbon chemical symbol (C). It is represented by ‘Z’.
LET’S DO it yourself….
Calculate
the molecular mass of the following in a.m.u?
N2O2, C2H2,
CH2, NO2
Calculate
the formula mass of the following
MgCl,
HCl, NO, CO
Calculate
the moles in 22 g of each?
O2,
C6H12O6, CH4, NH4Cl
Calculate
the mass in 5 moles of each?
Cl2,
N2, H2O, CH
Calculate
the number of atoms in 20 g of each?
Na,
Ca, Mg, N2